Electronegativity differs from electron affinity because electron affinity is the actual energy released when an atom gains an electron. What are the four most electronegative elements and what does electronegativity mean describe in your own words.
Definition Of Electronegativity Chemistry Dictionary
Electronegativity is important because it makes bonding between atoms possible.

. In your own words explain what the Partial Charges and Bond Character button display. In your own words describe the terms below. Electronegativity is a way to measure how much an atom attracts electrons in a chemical bond.
100 1 rating Transcribed image text. Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. Show More Sentences Find more words.
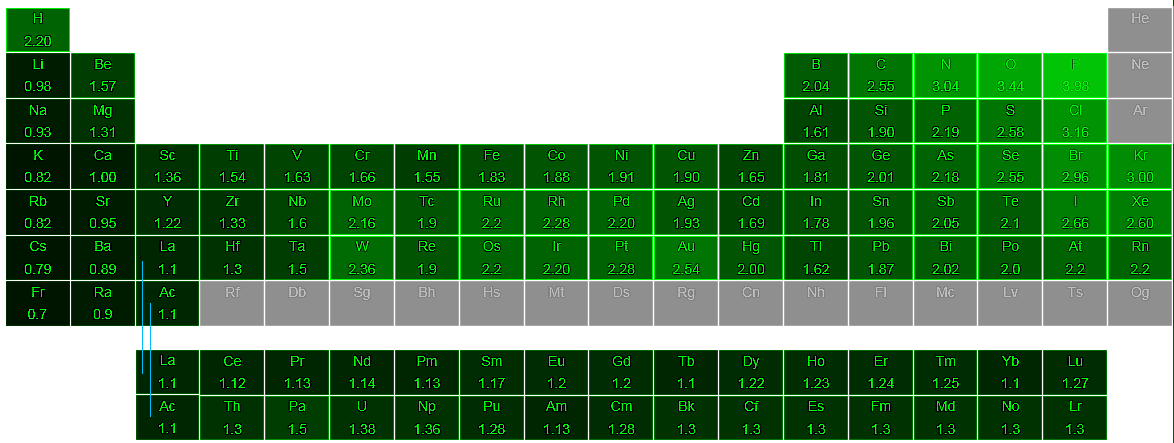
If the electrons of a bond are more attracted to one of the atoms because it is more electronegative the electrons will be unequally shared. The elements range in value from 07 caesium and francium the least electronegative to 40 fluorine the most electronegative. Describe the Octet Rule in your own words.
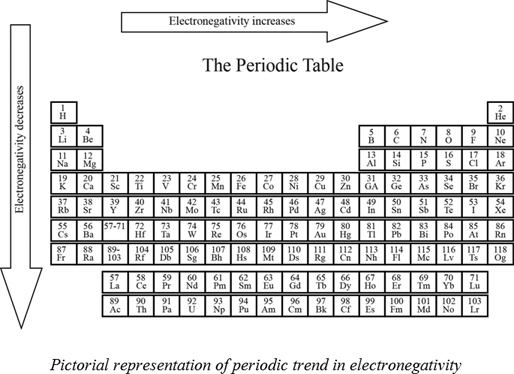
This number is closely linked to atomic number and radius. Describe the trend of electronegativity across the periodic table. Electronegativity is a property that describes the tendency of an atom to attract electrons or electron density toward itself.
5 points Partial Charges and Bond Character show that due to their electronegativity differences these two atoms are simultaneously willing to activate a covalent or ionic connection. The electronegativity of an atom or element is the strength of attraction for more electrons by that element. Electronegativity is a measure of an atoms ability to attract shared electrons to itself.
Electronegativity is a measure of the tendency of an atom to attract electrons or electron density within a bond. Electronegativity is affected by the atomic number and the distance between the valence electrons and its nucleus. Electronegativity describes the degree to which an atom attracts electrons in a chemical bond.
Describe in your own words the structure of solid sodium chloride and explain why its formula is NaCl. The higher the electronegativity the greater an atoms propensity to attract electrons. Electronegativity differences can be used to predict how shared electrons are distributed between the two nuclei in a bond.
The more strongly an atom attracts the electrons within its bonds the larger its electronegativity value. This is less applicable to compounds in which lead forms covalent bonds with elements of similar electronegativity such as carbon in organolead compounds. While other atoms have very little attraction for electrons.
We review their content and use your feedback to keep the quality high. Ionization energy- the amount of energy that an isolated gaseous atom in its ground electron state must absorb to discharge an electron making it. For example Potassium has a value on the Pauling scale of 08 which indicates a rather low electronegativity.
Electronegativity- the tendency of an atom to attract the shared pair of electrons b. An atoms electronegativity is affected by both its atomic number and the size of the atom. Electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond.
Use complete sentences to answer. It is a dimensionless property because it is only a tendency. The protons of an atom will always have an attraction for electrons.
The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity. In most cases the electrons found within a chemical bond have a greater attraction to one atom than to the other atom which creates a polar covalent bond. Electronegativity is a chemical property that measures the tendency of an atom to attract electrons towards itself.
Definition of electronegative. If atoms bonded together have the same electronegativity the shared electrons will be equally shared. The higher its electronegativity the more an element attracts electrons.
Electronegativity is not measured in energy units but is rather a relative scale. If the electronegativity difference is more than 17 the bond will have an ionic character. Ionic bonds form when one atom gives up one or more electrons to another atom.
Some atoms because of the electron configuration have more attraction than other atoms. On the periodic table electronegativity generally increases as you move from left to right across a period and decreases as you move down a group. Suppose A bond is formed as a result of chemical reaction between H and Cl and electrons are shared.
The higher the electronegativity is the more it attracts the electrons towards it. Movement of electrons from one element to another. Transcribed image text.
Now as Cl is more electronegative than H it will pull the electron of H towards itself more. Noun chemistry the tendency of an atom or radical to attract electrons in the formation of an ionic bond Etymologies from Wiktionary Creative Commons AttributionShare-Alike License electro- negativity Support Help support Wordnik and make this page ad-free by adopting the word electronegativity. Experts are tested by Chegg as specialists in their subject area.
It basically indicates the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs. Electronegativity refers to an atoms ability to attract the electrons present in a chemical bond or an atoms ability to attract electrons when that atom is part of a specific compound. Each atom contributes an equal number of electrons towards the bond formation.
The difference in the electronegativity of two atoms determines their bond type. Having a tendency to attract electrons Other Words from electronegative electronegativity i- ˌlek- trō- ˌne- gə- ˈti- və- tē noun First Known Use of. Electronegativity is the tendency of an atomelement to attract a shared pair of electrons towards itself.
Electronegativity is a measure of an atoms ability to attract the shared electrons of a covalent bond to itself. Chemistry the tendency of an atom or radical to attract electrons in the formation of an ionic bond. We review their content and use your feedback to keep the quality high.
Definition Of Electronegativity Chegg Com

What Is Electronegativity Brainly In

Solved In Your Own Words Define The Following Terms A Valence Electrons B Electronegativity C Bonddissociation Energy D Double Covalent Bond E Coordinate Covalent Bond

0 Comments